Abstract
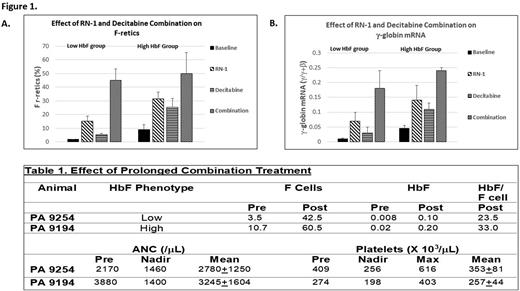
Elevated levels of Fetal Hemoglobin (HbF; α2γ2) reduce the symptoms associated with Sickle Cell Disease (SCD) and increase the lifespan of patients. An effective pharmacological therapy that increases HbF would advance the treatment of SCD. HbF levels are developmentally regulated by transcriptional repression of the γ-globin gene in adult erythroid cells. This is achieved by a co-repressor complex containing multiple enzymes that catalyze repressive epigenetic modifications of chromatin targeted to the γ-globin promoter by site-specific DNA binding proteins. Pharmacological inhibitors of repressive epigenetic enzymes within the complex including DNMT1, LSD1 (KDM1A), G9A, and HDACs increase HbF in cell line and animal models and SCD patients. Many of these drugs have been studied in Phase 1 trials, but only the DNMT inhibitor decitabine (DAC) has advanced to Phase 3 trials. The successful development of a pharmacological therapy to increase HbF for SCD has thus far been hindered by dose-limiting side effects including neutropenia, thrombocytopenia, and/or thrombophilia. The goal of this current work is to develop a pharmacological therapy employing a combination of drugs that target different repressive epigenetic enzymes within the co-repressor complex based on the hypothesis that administration of these drugs in combination will reduce the dose and/or time of exposure to any single agent to minimize adverse effects while still stimulating clinically meaningful HbF elevations. To test this hypothesis, baboons, long considered the best animal model to test the effect of HbF-inducing agents due to conservation of the structure and developmental regulation of the β-globin gene locus between baboons and humans, were treated with a combination of two drugs, DAC and RN-1, that inhibit DNMT1 and LSD1 respectively. Analysis of seven baboons used in our studies showed that they comprised two phenotypic groups differentiated by baseline pre-treatment HbF expression (Figure 1). Two baboons of each phenotypic group were chosen to study the effect of DAC and RN-1, alone and in combination, on HbF. Either RN-1 (0.25mg/kg/d) or DAC (0.5mg/kg/d), were administered subcutaneously individually or in combination to normal, non-anemic animals for 2 days. The effect on γ-globin expression was measured by flow cytometry analysis of F-retics and RT-PCR of γ-globin mRNA in peripheral blood at least 3X per week post-drug treatments. Each drug induced a significant increase in F-retics and peripheral blood γ-globin mRNA in both phenotypic groups that peaked on d8 (Figure 1) while differences in F-retics and γ-globin mRNA between the two groups were maintained. Combined administration (RN-1 (0.25mg/kg/d; DAC (0.5mg/kg/d); 2d) increased levels of F-retics and γ-globin mRNA to greater than additive levels (Figure 1) while the difference in γ-globin mRNA (p<0.02), but not F-retics, between the two groups was still observed. To assess the effect of sustained administration of the two drug combination on HbF expression and evaluate possible hematological toxicity, two normal baboons, one of the low HbF group (PA 9254) and one of the high HbF group (PA 9194), were treated weekly with 12 total courses of the DAC-RN-1 combination (DAC, 0.5mg/kg/d; RN-1, 0.25mg/kg/d; 2d/wk). F cells increased approximately 6 fold to 60.5% in the high HbF animal and >10 fold to 42% in the low HbF baboon (Table 1). HPLC analysis of globin chain expression showed that HbF (γ/γ+β) increased tenfold in each animal (Table 1). The calculated % HbF/ F cell values were 33% for PA 9194 (High HbF group) and 23.5% for PA 9254 (Low HbF group). No instances of moderate or severe neutropenia, thrombocytopenia, or thrombophilia were observed during the treatment period (Table 1). Decreased Hct, Hb, and RBCs were apparent in the high HbF group baboon (PA 9194) but not the low HbF group baboon (PA 9254). In conclusion, combinatorial administration of DAC and RN-1 increased HbF and F cell numbers in a greater than additive manner. Prolonged treatment of two normal, nonanemic baboons increased F cells and HbF to levels predicted to be clinically significant in the absence of neutropenia, thrombocytopenia and/or thrombophilia throughout the treatment period although a reduction in Hct, RBCs, and Hb was observed in one animal. These results strongly suggest additional drug combinations, dosing, and schedules to increase HbF for the treatment of SCD should be pursued.
Disclosures
Saunthararajah:EpiDestiny: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: intellectual property with royalty rights ; Novo Nordisk: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal